Enzyme Activity Factors:
An enzyme catalyzed reaction may be written as:
E is the enzyme
S is the substrate (reactant)
ES is the enzyme-substrate complex
P is the product
The Equation which
describes rate of reaction (r) as a function of substrate concentration (S) is
the Michaelis-Menton Equation.
Vmax is the function
of the concentration of the enzyme [E] and the Turnover Number of the enzyme.
Km is the
Michaelis-Menton constant and is determined experimentally, just as the rate
constant is for a rate law equation.
Remember, the Turnover
Number refers to the efficiency of the enzyme and is expressed as the number of
molecules of substrate converted to product per second.
The Turnover Number of
enzymes can range from 10 to 100,000 molecules per second, demonstrating the
effective catalytic nature of some enzymes.
Substrate Concentration
At low substrate
concentrations, each enzyme molecule is reacting with fewer substrate molecules
than is suggested by its turnover number. As the substrate concentration is
increased, each enzyme is able to locate and react with more substrate
molecules and the observed enzyme activity increases.
However, once the
turnover number is reached, the addition of more substrate does not further
increase the rate.
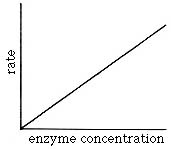
Enzyme Concentration
Under normal
biological conditions the substrate is present in a large excess (there is much
more substrate than enzyme). As long as this condition is maintained, the
addition of more enzyme results in a proportional increase in rate.
pH
Enzyme activity is
influenced by pH with each enzyme having an optimum pH. The optimum pH is the
pH at which the activity of a particular enzyme is at a maximum. The optimum
pH's for enzymes found in the body are matched to the pH of the biological
systems in which they operate. For example, pepsin, a digestive enzyme, has an
optimum pH of 1.5, the pH of the stomach.
At pH's far from the optimum pH,
enzyme activity can be reduced to nearly zero. If the reduction in activity is
reversible the enzyme has been denatured. If it is not reversible, the enzyme
has been digested.
Temperature
Just as each enzyme
has an optimum pH, each has an optimum temperature as well. Most human enzymes
have an optimum temperature about that of body temperature (98.6oF)
and are denatured or digested at extreme temperatures.
Presence of Cofactors
Some enzymes are
capable of catalytic activity by themselves. Others require the presence of an
additional substance called a cofactor to induce this behavior. If the cofactor
is an organic compound, it is called a coenzyme. If it is a metal ion, it is
called a metal ion activator. If a required cofactor is not present, the
catalytic activity of the enzyme is dramatically reduced.
Presence of Inhibitors
Inhibitors are
substances that reduce the rate of enzyme activity, usually by binding with the
enzyme and interfering with the formation of the enzyme-substrate complex.
While some heavy metals act as metal ion activators, they can also act as
enzyme inhibitors.









No comments:
Post a Comment